Sequential Parallel Comparison Design Increases Information 2.5x in Your Multi-center Clinical Trials
Our Placebo Response Reduction Strategy Optimizes Your Patient Information Efficiency
Our MGH team pioneered the game-changing SPCD trial design that cuts placebo response by half, allowing your trials to deliver compelling results using dramatically shorter timeframes due to smaller sample sizes. This design increases the number of informative subjects who receive active treatment and improves the power of your trial.
Why Placebo Response Saps Trial Success
In blind placebo-controlled trials, subjects on the placebo arm will sometimes display a perceived or actual response. High placebo response can cause a failure to show a difference between drug and placebo, resulting in a negative trial.
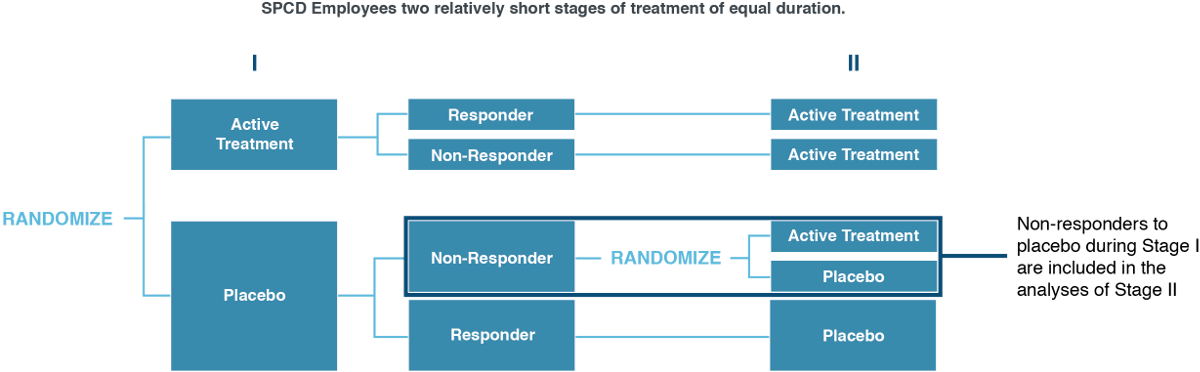
Anatomy of an SPCD Trial
The SPCD model uses two short stages of treatment, typically of equal duration. After Stage 1, only non-responders to placebo are included in the analyses of Stage 2. A special unbalanced randomization ratio between active treatment and placebo receivers ensures optimal patient information.
Putting SPCD in Action for Clinical Trials like Yours
Years of clinical trial experience have shown that SPCD can increase the power of a trial by approximately 10-25 percentage points over conventional designs—that’s 2.5X—or allow a sample size reduction of 20% – 50%.
SPCD conducts two stages of treatment and uses each patient at least once, and sometimes twice. Efficacy analysis is based on all data from Stage 1 and the Stage 2 data from re-randomized placebo non-responders in Stage 1.