Remote Interviews Optimize Best-Fit Patients for Your Multi-center Clinical Trials
Ensure Your Study Is a Good Test of the Intervention—With Lower Placebo Response
Our raters are both highly experienced psychiatrists and clinical researchers. They are well-versed in SAFER, one of the main methodologies utilized in the interviews. Our raters verify the diagnosis and appropriateness of the patient population, and ensure that appropriate patients are enrolled in your trials.
 What Makes SAFER Better? It Goes Beyond.
What Makes SAFER Better? It Goes Beyond.
The clinician-rated Massachusetts General Hospital SAFER interview facilitates assessment of important supplemental factors that go beyond conventional diagnostic criteria and symptom severity to identify patients who would be valid for clinical trials.
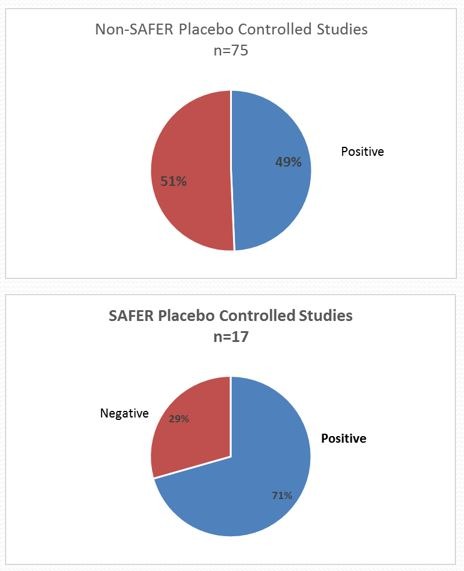
- SAFER increases the quality of your clinical trials by improving the separation of placebo responders from active drug responders and by restricting the sample to a tightly defined population.
- SAFER ensures the risk is minimized that factors unrelated to treatment may determine the patient’s outcome.
- When needed, our raters will present patient profiles to peers, who weigh in quickly to make a solid and speedy decision on the appropriateness of the patient.
The SAFER interview confirms that the illness is a specific state, and excludes patients with any symptoms that are nonspecific or not readily assessable.
Comparison of Placebo Controlled Studies with and without SAFER – 2009-2017
Where We Do Interviews
Countries We Conduct Interviews In:
- Argentina
- Australia
- Belgium (Flemish, French)
- Brazil (Portuguese)
- Bulgaria
- Chile
- Colombia
- Czech Republic (Czech, Slovak)
- Estonian/Russian
- Finland
- France
- Georgia
- Germany
- Hungary
- Italy
- Japan
- Lithuania
- Moldova
- Mexico
- Netherlands
- New Zealand
- Poland
- Romania (Romanian)
- Russia (Russian)
- Serbia (Serbian)
- Slovakia (Slovakian)
- South Africa (Afrikaans)
- South Korea
- Spain
- Sweden
- Switzerland (French)
- United Kingdom
- Ukraine (Ukranian)